Abstract
Background: MDS comprise a group of disorders characterized by bone marrow (BM) failure, cytogenetic and molecular alterations, and progression to acute myeloid leukemia (AML). Most lower-risk MDS patients (pts) eventually need chronic red blood cell (RBC) transfusions because of impaired hematopoiesis, resulting in iron overload (IO) that damages organ function and contributes to shortened survival. Though iron chelation therapy (ICT) has been consistently shown to improve outcomes in lower-risk MDS pts, most studies had limitations (eg retrospective analyses, registry studies, pts not stratified by performance status). As such, a prospective, controlled study of ICT and survival in Low/Intermediate (Int-1)-risk MDS pts was needed.
Aims: TELESTO (NCT00940602) was a Phase II randomized, double-blind study that evaluated event-free survival (EFS) and safety of deferasirox (DFX) vs placebo (PBO) in Low/Int-1-risk MDS pts.
Methods: Eligible pts were aged ≥18 yrs, had IPSS Low/Int-1-risk MDS (confirmed by BM examination within 6 months of study entry), serum ferritin (SF) >1000 ng/mL, transfusion history of 15-75 pRBC units, without cardiac, liver and renal abnormalities. Pts were randomized 2:1 to DFX (dispersible tablets; 10-40 mg/kg/day based on dosing guidelines) or PBO. Primary objective was to evaluate DFX and PBO for EFS, measured by a composite primary endpoint of time to first non-fatal event (related to cardiac and liver function and transformation to AML) or death, whichever occurred first; events were confirmed by an independent adjudication committee. With a planned sample size of 210 pts, no hypotheses were planned to be tested; statistical tests are exploratory, P values associated with treatment effect are nominal.
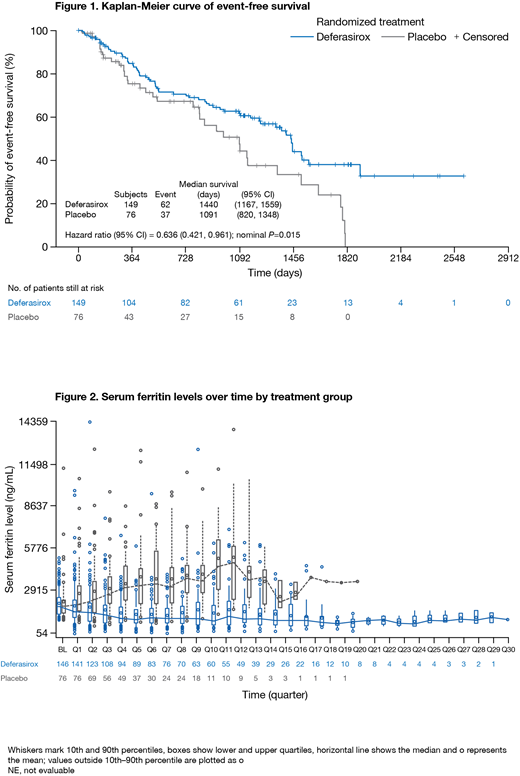
Results: 225 pts were randomized to DFX (n=149) or PBO (n=76); 72.4% had Int-1 MDS, 60.9% were male, mean age was 61.0 yrs; baseline characteristics were balanced, but more pts in the DFX arm were aged ≥75 yrs (25.5% vs 17.1%). DFX and PBO pts received a mean of 20.28 and 20.27 international units of pRBC transfusions 6 months prior to randomization. Median time on treatment was longer with DFX vs PBO (587.5 vs 370.5 days); 43.9% and 25.0% of DFX and PBO pts received treatment for ≥2 yrs. Median EFS was prolonged by 349 days with DFX (1440 days; 95%CI 1167-1559) vs PBO (1091 days; 95%CI 820-1348): 36.4% risk reduction in EFS with DFX (P=0.015 [Figure 1]). Estimated EFS at 3 yrs was 61.5% (95%CI 52.2-69.6) with DFX and 47.3% (95%CI 31.8-61.3) with PBO. Events that occurred first with DFX (n=62; 41.6%) and PBO (n=37; 48.7%) included: worsening cardiac function (1.3 vs 2.6%); hospitalization for congestive heart failure (0.7 vs 3.9%); liver function impairment (0.7 vs 1.3%); progression to AML (6.7 vs 7.9%); death (32.2 vs 32.9%). Robustness of the positive treatment effect with DFX was confirmed by various sensitivity analyses, including censoring pts with premature treatment discontinuation and subsequent ICT.
Median overall survival (OS) was 1907 days (95%CI: 1440-not estimable) with DFX and 1509 days (95%CI 1095-1804) with PBO; HR 0.832 (95%CI 0.54-1.28, P=0.200). ICT after study treatment discontinuation may have diluted any potential OS difference. SF declined over time with DFX and increased in the PBO arm (Figure 2).
Most frequently reported AEs (≥20% in either arm) were diarrhea, pyrexia, upper respiratory tract infection, cough and increased blood creatinine. After adjustment for exposure, rates were: diarrhea (24.7 vs 23.9%), pyrexia (21.8 vs 18.7%), upper respiratory tract infection (16.7 vs 22.7%), cough (12.6 vs 11.3%) and increased blood creatinine (15.9 vs 0.9%) in the DFX and PBO arms, respectively. Most frequently reported severe AEs (≥5% in either arm) were anemia, pyrexia, thrombocytopenia and lung infection. When adjusted for the different exposure time, the rate of all severe AEs per 100 pt-yrs was overall comparable.
Summary/conclusions: TELESTO is the first prospective, randomized study in Low/Int-1-risk MDS pts with IO to show ICT with DFX provides clinical benefit across multiple tissues, leading to longer EFS (including cardiac and liver events and transformation to AML) vs PBO. The safety profile was as expected, consistent with previous studies of DFX in adult MDS pts with IO. Considering the current treatment landscape, it is unlikely that a similar, randomized trial can be performed. These results support the use of DFX in Low/Int-1-risk MDS pts with IO.
Angelucci:Celgene: Honoraria, Other: Chair DMC; Vertex Pharmaceuticals Incorporated (MA) and CRISPR CAS9 Therapeutics AG (CH): Other: Chair DMC; Jazz Pharmaceuticals Italy: Other: Local ( national) advisory board; Roche Italy: Other: Local (national) advisory board; Novartis: Honoraria, Other: Chair Steering Comiittee TELESTO Protocol. Rodriguez:Novartis: Speakers Bureau. Dong:Novartis: Employment. Ghosh:Novartis: Employment. Bornstein:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal